Cetacea
Whales, dolphins, and porpoises
Michel C. Milinkovitch and Olivier Lambert

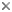
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Cetaceans (whales, dolphins and porpoises) form one of the most dramatically-derived group of mammals. Indeed, during their transition from the terrestrial to the aquatic environment, they experienced spectacular transformation of many biological systems and acquired a fusiform body shape giving modern families a superficial resemblance to fish. Extant cetaceans comprise approximately 87 species in 14 families and two sub-orders: Mysticeti (baleen-bearing whales) and Odontoceti (teeth-bearing whales) (Rice, 1998). Adult size ranges from less than 1.5 meters for some representatives of the Cephalorhynchus genus, to more than 30 meters for the largest blue whales. Cetaceans are ecologically very diverse as their habitats vary from coastal to pelagic, from tropical to polar, and from marine to fresh water, while their preys vary from planctonic crustaceans to fish, squids, and marine mammals.
Characteristics
The major morphological transformations related to the transition to the aquatic environment include:
- Loss of external hind limbs, although vestigial pelvic girdle bones are still present in all extant species;
- Paddle-like forelimbs with hyperphalangy and a non-rotational elbow joint;
- Torpedo-shaped body, with addition of vertebrae, reduction of the neck, and development of a horizontal caudal tail;
- Development of a thick blubber layer that prevents heat-loss;
- Telescoping of the skull, and posterior movement of the narial openings. The position of the external nares on the top of the skull allows cetacean to breathe while keeping the rest of the head underwater. The telescoping of the skull mostly involves the posterior extension of the bones of the elongated rostrum: maxillae, premaxillae, vomer and mesethmoid;
- Isolation of the earbones related to the development of underwater hearing and echolocation abilities.
Breathing
In mysticetes the nasal passages are separate tubes all the way to the external nares, whereas in odontocetes, the two nasal passages branch into a complex series of nasal sacs that eventually coalesce into a single blowhole. The sperm whale is however intermediate as it exhibits a sigmoidally-shaped blowhole formed by two nasal tubes that remain distinct from the bony nares to the top of the head: the anterior and posterior curves of the sigmoid blowhole represent the apertures of the right and left nares, respectively (rare examples of adult sperm whales with two distinct blowholes have even been reported). In the pygmy sperm whales (Kogiidae), the situation is only slightly different since the nasal passages remain discrete tubes until just proximal to the single blowhole.



Left: Toothed whales have a single blowhole. Right: Baleen whales have two blowholes. © Michel C. Milinkovitch

In the giant sperm whale, the nasal passages remain discrete tubes until just proximal to the single s-shaped blowhole.
© 1989 John E. Heyning.
Hearing and Echolocation
Echolocation is the ability to assess the environment (mostly, detecting obstacles, conspecifics, predators, and prey) by emitting sounds and listening to the echoes. Echolocation abilities have been conclusively demonstrated in delphinoids and so-called “river dolphins” (see below) and seem to be present in beaked and sperm whales as well. Although they can easily detect an object about the size of a ping-pong ball more than 50 meters away, dolphins also use echolocation to inspect objects at very short ranges (e.g., to assess shape, size, and structure). Dolphins produce short “clicks” whose frequencies (10 to 150 kHz) and intervals (as short as 1.2 milliseconds) are adjusted depending on the size and distance of the target (high frequencies dissipate more rapidly than low frequencies but allow the detection of smaller targets and a finer analysis of shape and structure). These sounds are produced by circulation of air in a complex system of facial sacs, reflected by the cranium, and focused into a beam by the melon, a fatty structure located in the forehead and serving as an acoustic lens. Mysticetes possess a small (Heyning 1989), possibly vestigial (Milinkovitch, 1995) melon.

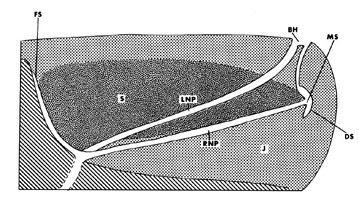
The melon is a a fatty structure located in the forehead of all cetaceans. In most toothed whales, it serves as an acoustic lens for echolocation sound production. The melon is shown in blue for the genera Globicephala, Inia, Ziphius, Balaena, and Physeter (from left to right). S, spermaceti organ.
© 1995 Michel C. Milinkovitch
The high-frequency echoes are received through the hollow mandible then transferred across fatty channels to the middle ear. Low-frequency sounds on the other hand are perceived through the auditory channels. The middle and inner ears of odontocetes are surrounded by an emulsion of mucus, oil, and air. The whole structure is enclosed into a dense, bony bulla. This high level of acoustic isolation greatly improves directional detection. The isolation mechanism is less complete in mysticetes, but the large distance between the two ears (simply because of the size of these animals) probably helps directional detection. Mysticetes exhibit a peculiar tympanic membrane (the finger glove) and seem to receive sounds through the auditory channels. It is unclear whether mysticetes are able to rely on the echoes of their low-frequency sounds.
Dorsal and anterior to the skull, sperm whales also have a large oily organ (called the “spermaceti organ” because early whalers thought the whitish oily substance was the whale semen) that possibly works as a biological ballast (Raven & Gregory 1933; Clarke 1970) or constitutes a secondary sexual character involved in acoustic display (Cranford 1999). Because of the high specialization of the giant sperm whale facial anatomy, it is unclear what structure in this species is homologous to the melon of other cetaceans. However, based on the comparison between the facial anatomy of dwarf and pygmy sperm whales, Heyning (1989) suggested the junk (a segmented tissue located below the spermaceti case and so-called because it is less rich in oil than the spermaceti itself) rather than the spermaceti organ as the most likely candidate. This hypothesis is supported by comparative CT-scan analyses (Cranford et al. 1996).
Origin of Cetacea
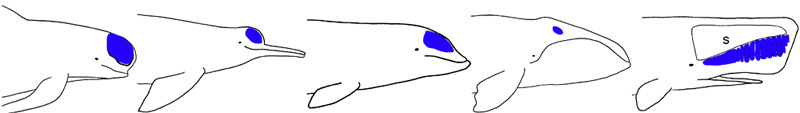
The oldest known cetaceans are Eocene in age, appearing around 55 million years ago. These primitive toothed cetaceans, lacking odontocetes and mysticetes cranial specializations, form the paraphyletic sub-order Archaeoceti. Representatives of this group have been found in Egypt, Nigeria, India, Pakistan, and USA and show a wide range of morphologies that prompted their classification into several families such as the Pakicetidae (early Eocene), Protocetidae (middle Eocene) and Basilosauridae (middle to Late Eocene). The most primitive taxa exhibited locomotory hind limbs (see Thewissen et al. 1994; Gingerich et al. 2001; Thewissen et al. 2001). The archaeoceti fossils illustrate the progressive reduction of hind limbs, adaptations of the ear for underwater hearing, and the expansion of the pterygoid sinus fossae in the basicranium leading to a better isolation of the ear bones.

Skeleton of Rhodocetus kasrani. Forelimbs were probably folded against the body during rapid swimming by pelvic paddling at the sea surface and during rapid swimming by pelvic paddling and caudal undulation when submerged. On land, Rodhocetus supported itself on hoofed digits II, III, and IV of the hands and on the plantar surfaces of the feet, and probably progressed somewhat like a modern eared seal or sea lion. Drawing from Gingerich et al. 2001. © 2001 American Association for the Advancement of Science
The origin of aquatic cetaceans has to be found among terrestrial mammals living in the early Tertiary. Analyses of neontological data have long suggested that cetaceans are closely related to ungulates (e.g., Flower, 1883; Prothero et al. 1988). These results were corroborated by paleontological data (Gingerich et al. 1990; Thewissen and Hussain, 1993; Thewissen, 1994). The close relationship between cetaceans and ungulates (and especially artiodactyls) was strongly supported by basically all molecular data published to date: from immunological (e.g., Boyden and Gemeroy, 1950; Shoshani, 1986) and DNA-DNA hybridization (Milinkovitch, 1992) studies, to analyses of mitochondrial and nuclear amino-acid and DNA sequences (e.g., Czelusniak et al. 1990; Milinkovitch et al. 1993; Gatesy, 1997; Milinkovitch et al. 1998, Gatesy et al. 1999).
The two main alternative hypotheses regarding the exact phylogenetic position of cetaceans within mammals are suggested respectively by morphological and molecular data. Morphological studies suggested that artiodactyls is the sister group to Cete -- i.e., a clade comprising cetaceans and a fossil group of carnivorous hoofed mammals: the mesonychians (see e.g. O'Leary and Geisler, 1999). On the other hand, molecular studies indicate that cetaceans are highly derived artiodactyls and that hippopotamids are their extant sister group. Although the nesting of cetaceans within artiodactyls (making the latter paraphyletic) was suggested by multiple studies (Goodman et al. 1985; Czelusniak et al. 1990; Irwin et al. 1991; Graur and Higgins, 1994; Gatesy et al. 1996; Smith et al. 1996; Gatesy, 1997; Montgelard et al. 1997) the statistical support or methods of analyses have been strongly criticized (e.g., Philippe and Douzery, 1994; Hasegawa and Adachi, 1996). This lead to a second series of molecular analyses with greatly increased taxon and gene sampling (Milinkovitch et al. 1998, Gatesy et al. 1999).
These latter analyses confirmed the monophyly of Cetartiodactyla (cetaceans,artiodactyls) and clarified its phylogeny. Furthermore, phylogenetic interpretation of retropositional events that lead to the insertion of short interspersed elements (SINEs) at particular loci in the nuclear genome of various artiodactyl and cetacean ancestors (Shimamura et al. 1997; Nikaido et al. 1999) lead to the same phylogenetic tree, suggesting that the paraphyly of artiodactyls is not the result of analytical problems related to the analysis of aligned nucleotide sequences (Milinkovitch and Thewissen, 1997; Shedlock et al. 2000). It is indeed the nature of SINE data that makes them so efficient in phylogeny inference: the likelihood of SINEs being independently inserted at the same locus in different lineages, or precisely excised in different lineages, seems virtually nil.
It remains to be understood why these results are in striking contradiction to the common interpretation of the available morphological data, namely, supporting artiodactyl monophyly (e.g., O'Leary and Geisler, 1999).
Note however, that the discrepancies between the morphological and molecular hypotheses have recently diminished. Indeed, recent discoveries of primitive whales from the families Pakicetidae and Protocetidae, from the early Eocene of Pakistan, allowed the description of skulls associated with hindlimbs (Thewissen et al. 2001; Gingerich et al. 2001). These fossils exhibit skull characters typical of primitive cetaceans and terrestrial hindlimbs with derived characters of artiodactyls. The morphology of the ankle (mainly the astragalus and calcaneum) of these terrestrial cetaceans suggest that Cetacea are the sister group of monophyletic Artiodactyla, and that [artiodactyls , cetaceans] form the sister group of mesonychians. In these new trees, cetaceans are therefore getting closer to artiodactyls but these analyses of morphological characters continue to support the monophyly of the latter group and, hence, to disagree with the molecular analyses.

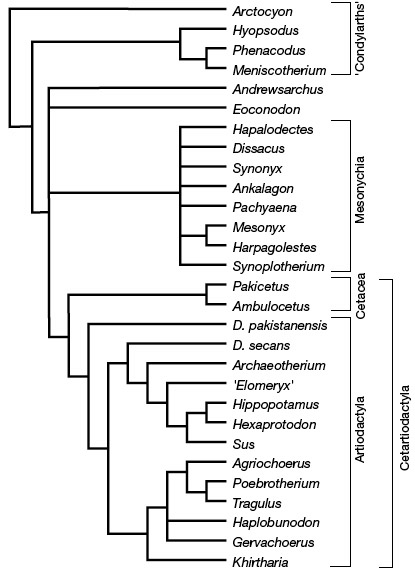
Relationships between Artiodactyla, Cetacea, and Mesonychia based on morphological data. From Thewissen et al. (2001).
Finally, Geisler and Uhen (2003) have performed maximum parsimony analyses of a matrix including substantially more taxa and morphological characters than previous analyses and found support for the sister-relationship between hippos and cetaceans, making molecular and morphological evidence for the phylogeny of these taxa much more congruent than previously thought.
Discussion of Phylogenetic Relationships
Some molecular studies based on mitochondrial DNA sequences (Milinkovitch et al. 1993; Milinkovitch et al. 1994) suggested that Physeteroidea (sperm whales) are more closely related to mysticetes than they are to other odontocetes. However, other molecular data (e.g., Nikaido et al. 2001; Gatesy et al. 1999) support the monophyly of toothed whales (Odontoceti). Recent analyses (Cassens et al. 2000) indicate that there is conflicting signal between the nuclear DNA and mitochondrial DNA data. Whether this conflict is due to differential lineage sorting or to misleading signal from one or several data set(s) remains to be investigated. This issue is further discussed in the Odontoceti (toothed whale) page.
Phylogenetic relationships among the families of extant cetaceans are still problematic and hypotheses on various nodes of the tree come from a variety of data: the morphology of extant taxa (including the soft anatomy) (e.g., Heyning, 1989), paleontological (e.g., Muizon 1991; Fordyce 1994; Geisler and Sanders 2003), and molecular evidence (e.g., Milinkovitch et al. 1993, 1994; Arnason and Gulberg, 1996; Cassens et al. 2000) or a combination of these (Milinkovitch 1995; Messenger and Mc Guire, 1998; Gatesy et al. 1999). This evidence will be discussed on relevant pages of cetacean subgroups.
References
Arnason, U. and A. Gullberg. 1996. Cytochrome b nucleotide sequences and the identification of five primary lineages of extant cetaceans. Molecular Biology and Evolution 13:407-417.
Boyden, A. and Gemeroy, D. 1950. The relative position of the Cetacea among the orders of Mammalia as indicated by precipitin tests. Zoologica, 35:145-151.
Cassens, I., S. Vicario, V. G. Waddell, H. Balchowsky, D. Van Belle, Wang Ding, Chen Fan, R.S. Lal Mohan, P. C. Simões-Lopes, R. Bastida, A. Meyer, M. J. Stanhope and Michel C. Milinkovitch. 2000. Independent adaptation to riverine habitats allowed survival of ancient cetacean lineages. Proc. Natl. Acad. Sci. USA, 97:11343-11347.
Clarke M.R. 1970. Function of the spermaceti organ of the sperm whale. Nature 228:873-874.
Cranford T. W. 1999. The sperm whale's nose: sexual selection on a grand scale? Marine Mammal Science 15:1133-1157.
Cranford T. W., Amundin M., and K.S. Norris. 1996. Functional morphology and homology in the odontocete nasal complex: implications for sound generation. J. Morph. 228:223-285.
Czelusniak, J., M. Goodman, B. F. Koop, D. A. Tagle, J. Shoshani, G. Braunitzer, T. K. Kleinschmidt, W. W. De Jong, and G. Matsuda. 1990. Pp. 545-572 In H. H. Genoways, Ed. Current Mammalology. Vol. 2. Plenum, New York.
Flower W.H. 1883. On whales, present and past and their probable origin. Proc. Zool. Soc., London, 1883:466-513.
Fordyce R.E. 1994. Waipatia maerewhenua, new genus and new species (Waipatiidae, new family), an archaic late Oligocene dolphin (Cetacea: Odontoceti: Platanistoidea) from New Zealand. Proc. Sand Diego Soc. Nat Hist. 29:147-176.
Gatesy, J. 1997. More DNA Support for a Cetacea/Hippopotamidae Clade: The Blood-Clotting Protein Gene y-Fibrinogen. Mol. Biol. Evol. 14(5):537-543.
Gatesy, J. 1998. Molecular evidence for the phylogenetic affinities of Cetacea. pp. 63-112 in: Thewissen J.G.M. (ed.), The Emergence of Whales: Evolutionary Patterns in the Origin of Cetacea , Plenum, New York.
Gatesy, J. , C. Hayashi , M. Cronin, and P. Arctander. 1996. Evidence from milk casein genes that cetaceans are close relatives of hippopotamid artiodactyls. Mol. Biol. Evol. 13:954–963.
Gatesy, J., Milinkovitch, M. C., Waddell, V. and Stanhope, M. 1999. Stability of Cladistic Relationships between Cetacea and Higher Level Artiodactyl Taxa. Systematic Biology 48:6-20.
Geisler, J.H. and M. D. Uhen. 2003. Morphological support for a close relationship between hippos and whales. Journal of Vertebrate Paleontology 23:991-996.
Geisler, J. H. and Sanders, A. E. 2003. Morphological evidence for the phylogeny of Cetacea. Journal of Mammalian Evolution 10(1-2):23-129.
Gingerich, P. D., B. H. Smith, and E. L. Simons. 1990. Hind limbs of Eocene Basilosaurus: evidence of feet in whales. Science 249:154-157.
Gingerich, P. D., M. ul Haq, I. S. Zalmout, I. H. Khan, and M. S. Malkani. 2001. Origin of whales from early artiodactyls: hands and feet of Eocene Protocetidae from Pakistan. Science 293:2239-2242.
Goodman, M., J. Czelusniak, And J. E. Beeber. 1985. Phylogeny of Primates and other eutherian orders: a cladistic analysis using amino acid and nucleotide sequence data. Cladistics 1:171-185.
Graur, D. and D.G. Higgins. 1994. Molecular evidence for the inclusion of cetaceans within the order artiodactyla. Mol. Biol. Evol. 11:357-364.
Hasegawa, M. and J. Adachi. 1996. Phylogenetic position of cetaceans relative to artiodactyls: Reanalysis of mitochondrial and nuclear sequences. Mol. Biol. Evol. 13:710-717.
Heyning, J. E. 1989. Comparative facial anatomy of beaked whales (Ziphidae) and a systematic revision among the families of extant Odontoceti. Cont. Sci. Nat. Hist. Mus. Los Angeles 405:1-64.
Irwin, D. M., T. D. Kocher, and A. C. Wilson. 1991. Evolution of the cytochrome b gene of mammals. J. Mol. Evol. 32:128-144.
May-Collado, L. and I. Agnarsson. 2006. Cytochrome b and Bayesian inference of whale phylogeny. Mol. Phylogenet. Evol. 38:344–354
Messenger, S. L. and J. A. McGuire. 1998. Morphology, molecules, and the phylogenetics of cetaceans. Systematic Biology 47:90-124.
Milinkovitch, M. C. 1992. DNA-DNA Hybridizations Support Ungulate Ancestry of Cetacea. J Evol Biol, 5:149-160.
Milinkovitch, M. C. 1995. Molecular phylogeny of cetaceans prompts revision of morphological transformations. Trends in Ecology and Evolution 10:328-334.
Milinkovitch, M. C., M. Bérubé, and P. J. Palsbøll. 1998. Cetaceans Are Highly Specialized Artiodactyls. pp. 113-131 in: Thewissen J.G.M. (ed.), The Emergence of Whales: Evolutionary Patterns in the Origin of Cetacea. Plenum, New York.
Milinkovitch, M. C., A. Meyer, and J. R. Powell. 1994. Phylogeny of all major groups of cetaceans based on DNA sequences from three mitochondrial genes. Molecular Biology and Evolution 11:939-948.
Milinkovitch, M. C., G. Ortí, and A. Meyer. 1993. Revised phylogeny of whales suggested by mitochondrial ribosomal DNA sequences. Nature 361:346-348.
Milinkovitch, M. C., and Thewissen, J.G.M. 1997. Eventoed Fingerprints on Whale Ancestry. Nature 388:622-624.
Montgelard, C., F.M. Catzeflis and E. Douzery. 1997. Phylogenetic Relationships of Artiodactyls and Cetaceans as Deduced from the Comparison of Cytochrome b and 12s rRNA Mitochondrial Sequences. Mol. Biol. Evol. 14(5):550-559.
Muizon, C. de. 1990. A new Ziphiidae (Cetacea) from the Early Miocene of Washington State (USA) and phylogenetic analysis of the major groups of odontocetes. Bulletin du Muséum National d'Histoire Naturelle, Paris 4 C, (3-4): 279-326.
Nikaido M., A.P. Rooney,and N. Okada. 1999. Phylogenetic relationships among cetartiodactyls based on insertions of short and long interpersed elements: Hippopotamuses are the closest extant relatives of whales. Proc. Natl. Acad. Sci. USA 96, pp. 10261–10266.
Nikaido, M., Matsuno, F., Hamilton, H., Brownel, R. L. Jr, Cao, Y., Ding, W., Zuoyan, Z., Shedlock, A. M., Fordyce, R. E., Hasegawa, M., and Okada, N. 2001. Retroposon analysis of major cetacean lineages: the monophyly of toothed whales and the paraphyly of river dolphins. Proceedings of the National Academy of Sciences 98 (13): 7384-7389.
O'Leary, M.A. and J.H. Geisler. 1999. The position of Cetacea within Mammalia: phylogenetic analysis of morphological data from extinct and extant taxa. Syst. Biol. 48: 455-490.
Philippe H. and Douzery E. 1994. The pitfalls of molecular phylogeny based on four species as illustrated by the Cetacea/Artiodactyla relationships. J. Mammal. Evol. 2: 133-152.
Price, S. A., O. R. P. Bininda-Emonds, and J. L. Gittleman. 2005. A complete phylogeny of the whales, dolphins and even-toed hoofed mammals (Cetartiodactyla). Biol. Rev. 80:445–473.
Prothero D., Manning E. and Fisher M. 1988. The phylogeny of the unglates. IN: The phylogeny and classification of the tetrapods, volume 2: Mammals (Ed. by M. Benton), pp. 201-234. CXlarendon Press, Oxford.
Raven H. C. and W.K. Gregory. 1933. The spermaceti organ and nasal passages of the sperm whale (Physeter catodon) and other odontocetes. American Museum Novitates 677: 1-18.
Rice, D. W. 1998. Marine mammals of the world. Systematics and distribution. Society for Marine Mammalogy, Special Publication, 4, Lawrence, Kansas, 231 pp.
Rothausen, K. 1968. Die systematische Stellungsder europaischen Squalodontidae (Odontoceti, Mammalia). Palaeontologische Zeischrift 42: 83-104.
Shedlock A. M., M.C. Milinkovitch, and N. Okada. 2000. SINE Evolution, Missing Data, and the Origin of Whales. Syst. Biol. 49(4):808–817.
Shimamura M., H. Yasue, K. Ohshima, H. Abe, H. Kato, T. Kishiro, M. Goto,I. Munechikak and N. Okada. 1997. Molecular evidence from retroposons that whales form a clade within even-toed ungulates. Nature 388: 666-670.
Shoshani J. 1986. Mammalian Phylogeny: Comparison of Morphological and Molecular Results. Mol. Biol. Evol. 3(3):222-242.
Smith, M., M. Shivji, V. Waddell, and M. Stanhope. 1996. Phylogenetic evidence from the IRBP gene for the paraphyly of toothed whales, with mixed support for Cetacea as a suborder of Artiodactyla. Mol. Biol. Evol. 13:918-922.
Thewissen, J. G. M. 1994. Phylogenetic aspects of cetacean origins: a morphological perspective. J. Mamm. Evol. 2: 157-183.
Thewissen, J. G. M. and S. T. Hussain. 1993. Origin of underwater hearing in whales. Nature 361:444-445.
Thewissen, J. G. M., S. T. Hussain, and M. Arif. 1994. Fossil evidence for the origin of aquatic locomotion in archaeocete whales. Science 263:210-212.
Thewissen, J. G. M. , E. M. Williams, L. J. Roe and S. T. Hussain. 2001. Skeletons of terrestrial cetaceans and the relationship of whales to artiodactyls. Nature 413: 277-281.
Wada, S., Oishi, M., and Yamada, T. K. 2003. A newly discovered species of living baleen whale. Nature 426: 278-281.
Zimmer, C. 1998. At the Water's Edge: Fish with Fingers, Whales with Legs, and How Life Came Ashore but Then Went Back to Sea. Touchstone, New York.
Information on the Internet
- Order Cetacea. Animal Diversity Web. University of Michigan Museum of Zoology.
- Whales | Tohorā: Whale Lab. Museum of New Zealand Te Papa Tongarewa
- BioQUEST: Whippo Problem Space
- Lesson: Whale Evolution. Evolution & the Nature of Science Institutes, Indiana University.
- American Cetacean Society.
- National Marine Mammal Laboratory (NMML).
- Canada's Marine Mammals. University of Guelph.
- Indonesia Oceanic Cetacean Program.
- Center for Oceanic Research and Education.
- WhaleNet at Wheelock College.
- Institute of Cetacean Research.
- Oceania Project: Caring for Whales, Dolphins & the Oceans.
- Whale-Watching-Web.
- Azorian Whale Watching Base.
- Whale Dreams: Spotting Whales in Australia. Environment Australia - Biodiversity Group.
- The Whale Center of New England.
- Marine Mammal Program. U. S. National Marine Fisheries Service Office of Protected Resources.
Early Cetacea Fossils:
- Whale Origins. J. G. M. "Hans" Thewissen, Northeastern Ohio Universities.
- Research on the Origin and Early Evolution of Whales (Cetacea). Philip D. Gingerich, University of Michigan.
- Mark D. Uhen's Research in Vertebrate Paleontology
- Whales | Tohorā: Whale Lab: Evolution. Museum of New Zealand Te Papa Tongarewa
- Whale Evolution. PBS Evolution Library.
- Whales: From So Humble A Beginning.... The Loom, Carl Zimmer.
- The Origin of Whales and the Power of Independent Evidence. Raymond Sutera.
- Ambulocetus as a Fossil Transitional. Lenny Flank, Creation "Science" Debunked.
- Cetaceans (Whales and Dolphins). UCMP Berkeley.
Title Illustrations

| Scientific Name | Stenella coeruleoalba |
|---|---|
| Location | Mediterranean Sea, between France and Corsica. |
| Copyright |
© 1991 Michel C. Milinkovitch

|
About This Page
Michel C. Milinkovitch

Genetics & Evolution, University of Geneva
Olivier Lambert

Royal Institute of Natural Sciences (Belgium)
Correspondence regarding this page should be directed to Michel C. Milinkovitch at
Page copyright © 2006 Michel C. Milinkovitch
All Rights Reserved.
- Content changed 07 August 2006
Citing this page:
Milinkovitch, Michel C. and Olivier Lambert. 2006. Cetacea. Whales, dolphins, and porpoises. Version 07 August 2006 (under construction). http://tolweb.org/Cetacea/15977/2006.08.07 in The Tree of Life Web Project, http://tolweb.org/






 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site